#MakeScienceCoolAgain
12/14/2017
By: Christine Clutton S u n d a y night I found myself wearing a white lab coat and protective goggles while holding a test tube with boiling green chemicals spilling out . . . metaphorically speaking that is. Really I was wearing my pajamas and Warby Parker glasses while holding a Brita filter in one hand and baking soda in the other. Albeit that doesn’t sound as scientific as the first scenario, for me, it was a step into the wild unknown of the science world. A world I intentionally tried to avoid my whole life. A world I thought was filled with braces, spectacles, and a void of understanding social cues. I know, I’m stereotyping here. You’d think that stereotype would have gone away after marrying my husband, who by all means, is a scientist. But it hadn’t gone away. And when Jon told me, two months ago, that he wanted to write science related topics in our blog, I literally cringed and hoped he would forget he asked. He didn’t forget. Fast-forward to Sunday night when Jon and I were sitting on our black-and-white-checkered kitchen floor laughing at how our science experiment showed why our coffee at home had failed to hold up to our standards. We finally discovered why our home coffee tasted like shit. I wrote three blogs about what we did to fix our coffee, and all three blog topics really did improve our overall cup quality; but, we were still missing something in our morning coffee. Our experiment Sunday night showed we were missing one crucial element to our water. No amount of brewing devices or varying roasts would have figured out our missing ingredient. The only thing that could have shown us . . . was . . . science. Flipping science. I am now a converted scientist. I ordered an engraved plaque in the mail to hang on my wall. It will read. “Christine Clutton, Scepticist to Scientist. Converted 2017” |
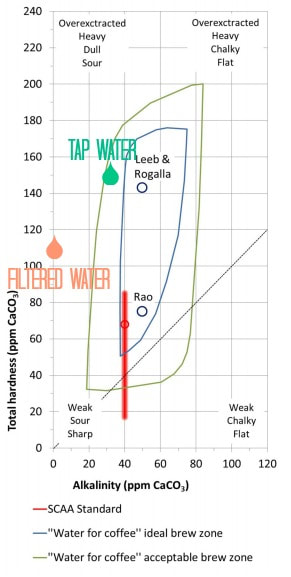
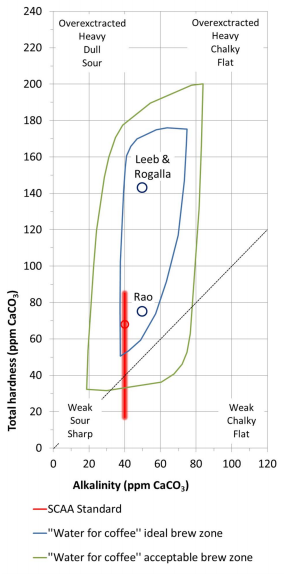
| We wanted to see if we could tell a difference in the coffee if the water had a buffer. So we made our own water by adding a buffer. We added a tiny amount (0.02g) of baking soda (buffer) to 800 grams of our double filtered water. We tested the water with the KH solution and got to three drops, or 51ppm of alkalinity, which is in the ideal brewing range as per our graph. We then did a blind experiment comparing coffee brewed with our regular double filtered water and one brewed with our double filtered water with added buffer (baking soda). Jon knew which water was which. I did not. We brewed THIS seasonal Kenyan from Thou Mayest in the same kalita wave, using the same water-to-coffee ratio, and the same water temperature. |
After they brewed, we ensured both coffees were at the exact same temperature (140 degrees), and then drank the coffee and graded on flavor, mouthfeel, acidity, and aroma. We wrote down our thoughts separately without sharing.
We waited five minutes and re-tasted at a lower coffee temperature (the colder the coffee the more you can taste the good, bad, and ugly in coffee).
It was really that second tasting that made it clear as day to me.
There was no question. The blue cup had a much higher, more uncomfortable acidity, and pulled out more tomato flavors that made me pucker. It had a slightly sour-lemony after taste. The pink cup, had nice nutty flavors, low but good acidity, and almost no vegetal characteristics. It smelt like sweet cocoa and was full bodied on my tongue.
It was really that second tasting that made it clear as day to me.
There was no question. The blue cup had a much higher, more uncomfortable acidity, and pulled out more tomato flavors that made me pucker. It had a slightly sour-lemony after taste. The pink cup, had nice nutty flavors, low but good acidity, and almost no vegetal characteristics. It smelt like sweet cocoa and was full bodied on my tongue.
I pointed to the pink cup and said to Jon, “this one has the buffer.” Sure enough I was right. The pink cup of coffee, which was literally the exact same as the blue cup, other than the added buffer, was superior in flavor, mouthfeel, acidity, and aroma. Hands down in every category.
So here I am. Drinking a cup of coffee brewed with the baking soda water solution and loving my life. I used to think science was lame. But I write to you today to tell you it is not. It’s the Ryan Gosling of school subjects and professions. Science can get you to the moon, to your next destination, to your best cup of coffee, to your best date night. If you are wanting to recreate this experiment at home, all you need is that $7 test from amazon. You may not need the baking soda depending on your results from the test. You can use Henden's ideal brewing chart (below) to see where your home water sits in the graph and make decisions about filtering, adding buffer, etc from there. Feel free to contact us or comment below if you have any questions or try the experiment at home!
E n j o y t h e d i s c o v e r y .
Happy Brewing,
Christine Clutton
Owner/Operator/Barista
So here I am. Drinking a cup of coffee brewed with the baking soda water solution and loving my life. I used to think science was lame. But I write to you today to tell you it is not. It’s the Ryan Gosling of school subjects and professions. Science can get you to the moon, to your next destination, to your best cup of coffee, to your best date night. If you are wanting to recreate this experiment at home, all you need is that $7 test from amazon. You may not need the baking soda depending on your results from the test. You can use Henden's ideal brewing chart (below) to see where your home water sits in the graph and make decisions about filtering, adding buffer, etc from there. Feel free to contact us or comment below if you have any questions or try the experiment at home!
E n j o y t h e d i s c o v e r y .
Happy Brewing,
Christine Clutton
Owner/Operator/Barista
Comments
THE WILD WAY CONTRIBUTING WRITERS:
CHRISTINE CLUTTON
boss lady
JON CLUTTON
coffee scientist
Science meets creativity in our coffee blog written by The Wild Way owners











 RSS Feed
RSS Feed