Intro To Total Hardness
12/7/2017
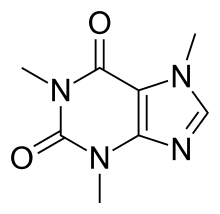
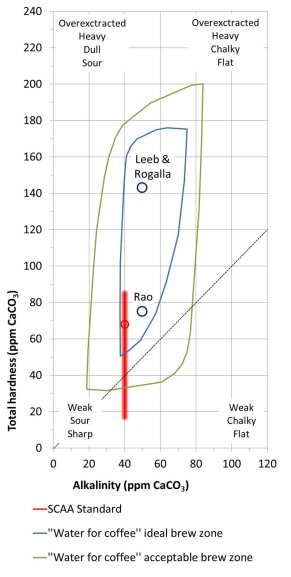
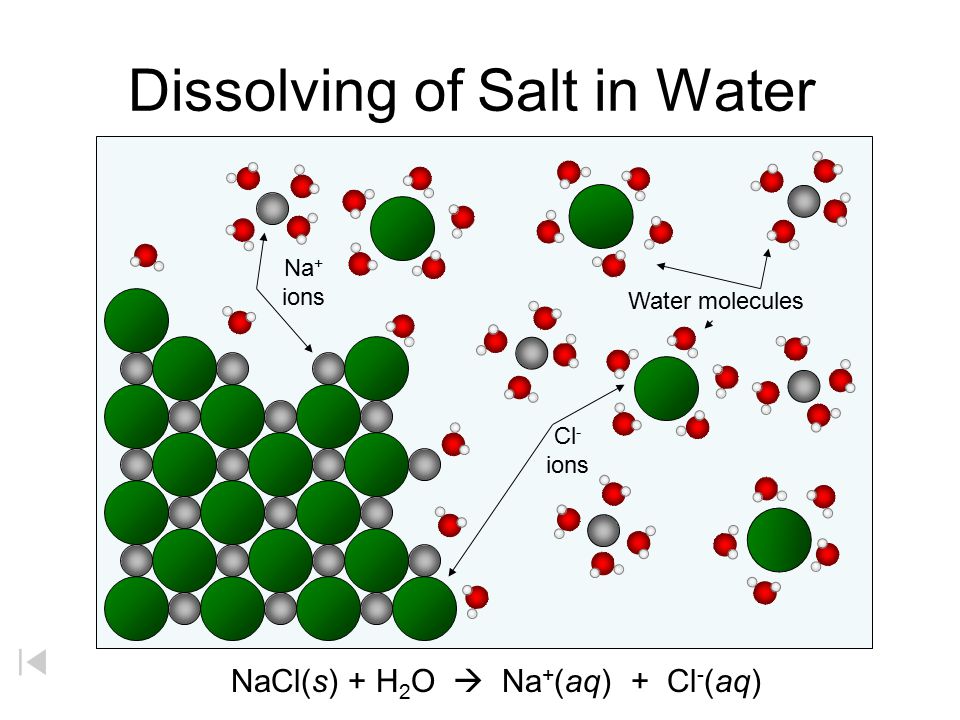
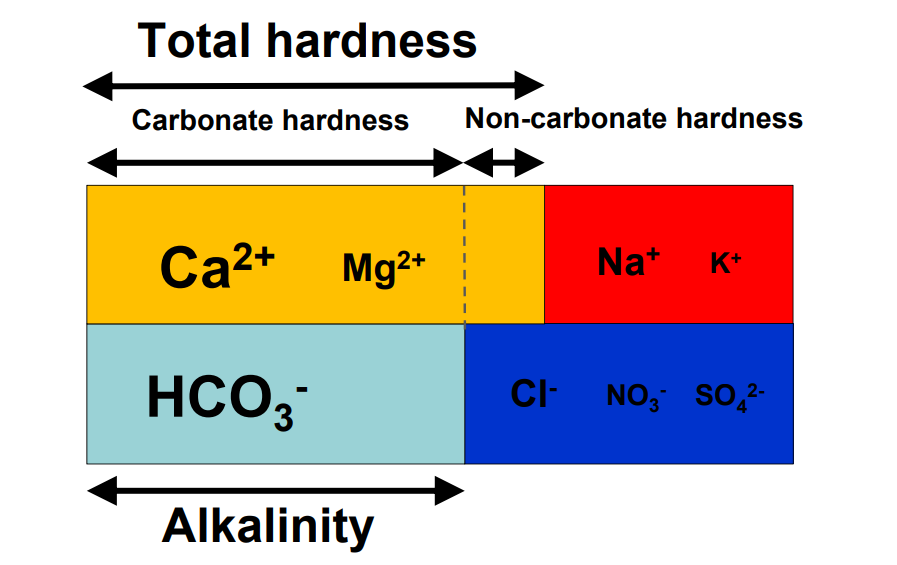
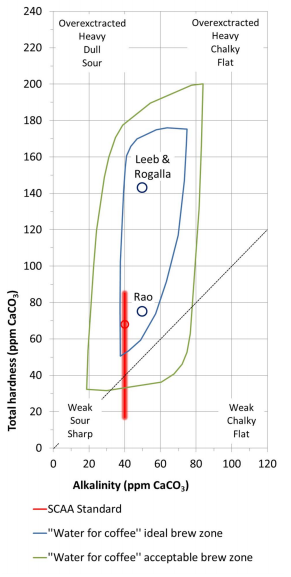
By: Jon CluttonOkay. I take responsibility. I took us straight into the deep end last week. We dove right into molecules that look like this. That can be confusing AF for those of us who haven't taken a science class since high school. I might have been a bit overzealous. But I swear it was with good intentions. You see, I LOVE science and I feel it gets a bad rap. People think it's reserved only for the nerds, the PhDs, and the liberals. When really, ALL people need science and can learn a lot from it. I want the science section of this blog to be one where we all feel comfortable looking at data. I want us to look, understand, and not be intimidated by the data. Being able to look at data and not be intimidated is a great skill. It's a real skill that we can use every single day - sourcing foods, whether or not to trust online articles, and analyzing the latest documentary you watched on netflix. We should make decisions based on the weight of the evidence. So, on this blog, I want to be grounded in data. I don't want there to be conjecture or philosophizing. I don't want things to be dumbed down. I don't want to "interpret" anything. I want for all of us to look at a study, look at the crazy graphs, and be like "ya, I understand that." Because we can. We can in coffee. We can in health. We can in climate change. We are capable. We can look at the data for ourselves and find answers. The only places we can't are in subjects like biophysics and complicated materials sciences. . . but we have friends for that. Or at least Christine and I do. If you need some of those friends, we can share them with you. They're real nice. (Jose Luis Olmos Jr., Adrianne Spencer, and David Spencer). #scienceforeveryone #dataisreal #scienceisreal With my small rant being out of the way, let's take a step back and actually build a foundation so that we can all think about coffee a little bit differently and a little more sciencey. Here's the plan for the next four weeks. Week 1 - Introduce General Hardness (this one) Week 2 - Introduce Alkalinity Week 3 - Put It All Together Week 4 - Collect Our Own Data I want to just put a little bit of data in front of you. Let's just let that marinate for a few seconds. (Pause for marinating). You might be thinking, "what the heck even is this?" This is a graph of how the relationship between water alkalinity and total hardness impacts the quality of a brewed cup of coffee. In other words, this is a graph describing the important relationship between extraction capacity and buffering capacity. In other, other words, this graph explains how to make good coffee based on lots of data. By the end of the four weeks we will be able to understand this graph and how it can help us. Keep this in the back of your mind. Ion IntroAn ion is a charged particle. Table salt is composed of sodium and chloride (NaCl). When you salt your water before making your pasta, the NaCl is dissolved into water and it splits into Na+ and Cl- molecules. Na+ and Cl- are ions. They are positively and negatively charged, respectively. If you were to take a super duty microscope and zoom in on the water in your pot, it would look something like this, with the charged ions being surrounded by the water. There are many different ions present in water. The few that we care about for today are magnesium (Mg2+), calcium (Ca2+), and forms of carbonate (H2CO3, HCO3-, CO32-). Total HardnessIf you remember back to our last blog, magnesium and calcium are the ions responsible for extracting a lot of the good stuff out of the coffee bean. They are important for making coffee tasty. When we refer to total hardness, we refer to calcium and magnesium, as you can see on this handy chart below. The amount of dissolved calcium and magnesium make up the total hardness. So, let's go back to the graph that we showed earlier. Plotted on the y-axis (vertical axis) is total hardness (think magnesium and calcium). We can think of total hardness as the extraction capacity of the water. The higher the total hardness, the more the water can pull from the coffee bean. In the top left corner of the image the tasting notes are overextracted, heavy, dull, and sour. The key there is overextracted. That's why I bolded it. Christine probably would have even made it a different color altogether. So I'm going to do that too. OVEREXTRACTED. At those higher ranges of total hardness, the water is pulling TOO MUCH from the coffee beans. It's even pulling the molecules that do not taste good. When you taste coffee that has been overextracted, it has a linger on your tongue. It has a heavy mouth feel to it because of the high number of dissolved solutes the total hardness pulled. You will also notice another tasting note in the top right hand corner - sour. That comes from the acids. The high total hardness pulled all of the acids into the cup of coffee, leading to a very acidic cup. Think sour patch kids in coffee form. It's not great. There was no buffer to help balance out the acidity. This is where the alkalinity comes in. We will get into that next week. My wife found a good metaphor for total hardness. Christine's Interpretation: Pie is fantastic. Delicious pie is just unmatched. I mean, who doesn't want to eat pie? I can't name anyone. Seriously. Not a single human. My grandma makes a mean rhubarb pie. You would think, because pie is so damn good, you could eat a crap ton of pie (any flavor - pick your favorite) and never get tired of it. But we all know that's not true. There's a point, maybe it's your third, maybe fourth, or maybe your fifth slice when you no longer want to take another bite. It's just too heavy. You start to pick up on weird flavors in the filling. The perfection of the crust no longer seems ideal. All you want is a piece of kale and a nice nap. That's when you know you've past your ideal total pie level. In the same way, we want magnesium and calcium, but at some point, you get too much of a good thing, and it turns bad. That's when you've past your ideal Total Hardness Level. Key TakeawaySWe are building a foundation, so let's talk about the key takeaways from this first foundation level. Total hardness refers to the amount of magnesium and calcium in water, which we can think of as the water's extraction capacity. Total hardness = extraction capacity. Too much of a good thing, can be a bad thing. And, perhaps most importantly, If you don't like pie, then something may be wrong with you. Jon Clutton
|
THE WILD WAY CONTRIBUTING WRITERS: |



 RSS Feed
RSS Feed