An Ion Took A Compound on A Date
11/30/2017
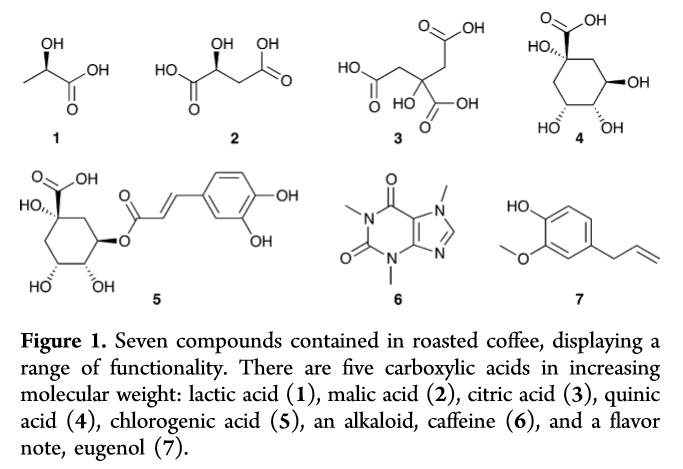
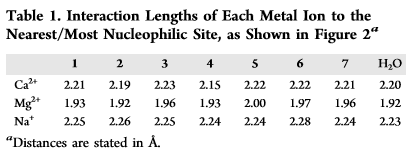
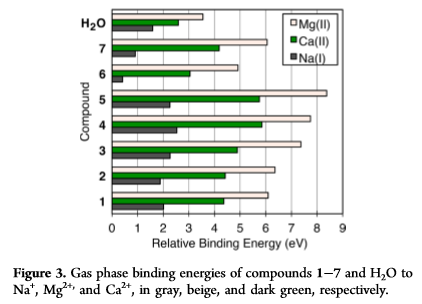
By Jon CluttonOne of my goals as a barista and scientist is to be able to encourage creative and curious minds in coffee to read the science behind what makes their drinks taste incredible. Ultimately, our goal is to craft incredible coffee. Looking into the science helps us do just that. There are two ingredients in all coffee drinks. Coffee beans and water. But mostly, it's just water. So I am going to write the next few blogs on water. I figured we could start by building a foundation on water by reviewing an article by an incredible mind in the coffee world, Christian Hendon et al. (2014). There may only be two ingredients to coffee, but when you break down each of those ingredients into their molecular level, then things can get real interesting. If things get too "heady" feel free to read Christine's interpretations at the bottom of each section in orange. This is her explaining the science in her own words. Background"The Role of Dissolved Cations in Coffee Extraction" by Hendon et al (2014) was the paper that sparked a lot of the interest in how water impacts spro and joe. It is the reason companies like Third Wave Water can do what they do and the reason you see a bunch of blog posts popping up about homemade water recipes i.e this one from Matt Perger at Barista Hustle. Dr. Christopher Hendon, the author of this paper, is an assistant professor in computational chemistry at the University of Oregon. So in other words, he smart. In these last few years he has spearheaded some serious coffee science innovation, both with his book, Water for Coffee, which expands on the topics covered in his 2014 paper and with a paper on grinding temperature published in Nature Scientific Reports - Uman et al. (2016). Coffee is just water and coffee beans. That’s it. By mixing water and coffee beans, whatever the brewing device might be, you pull stuff from the coffee beans into the water, and BOOM, coffee. Our ability to pull stuff is called extraction. I think that this concept is pretty easy to understand using tea. Think about when you steep a tea bag. The longer you leave the tea bag steeping in the water, the stronger the tea, i.e. the more stuff you’ve pulled out, i.e. the higher the extraction. Same thing goes for a french press or a cold brew. The longer you leave it brewing, the higher the extraction. However, not everything in coffee beans tastes good. You don’t want to pull out everything. So the battle for roasters is, how do I roast to accentuate the good stuff in this coffee, and the battle for baristas is, how do I brew so that I extract the good stuff from this coffee. This is what Hendon tackles. What does water extract and how? Hendon et al. (2014) proposes looking at the interactions between molecules commonly found in coffee and ions in water through computational chemistry. He narrows his focus down to three ions - calcium, magnesium and sodium - and seven compounds - lactic acid, malic acid, citric acid, quinic acid, chlorogenic acid, caffeine, and eugenol. (See Hendon et al. 2014 Table 1 below) Below is a fancy scientific way of showing these compounds. If it looks confusing, just pretend they are drawings of partial honeycombs for now. Christine's Interpretation: Coffee is mostly water. "Extraction" is a term that means "pulling things out of the coffee bean into the water". You can over and under extract coffee leaving not so pleasant tastes. You're looking to create the perfect cup by extracting the good, and leaving out the bad. MethodsHendon uses software to model the environment of brewing coffee at a molecular molecular level. (Hendon goes into his methods in the paper, and he goes into the basic science behind those methods in his book Water for Coffee. If you're interested I'd suggest reading him for more information). Using this model, Hendon determines the strength of the interaction between each ion, the seven chosen coffee compounds, and water. You can think of the interaction as the pull. The stronger the interaction, the stronger the pull. With this information Hendon can discuss the extraction capacity of each ion. The stronger an ion interacts with a coffee compound, the more likely it is to pull the compound into the cup of coffee. . . the higher the extraction. Christine's interpretation: Imagine you have three female roommates and you want to set them up with one of your seven guy friends. You'd put each roommate together with each guy and see how they interact. The stronger a roommate interacts with one of the guy friends, the more likely the relationship would actually work and produce something good. So homeboy Hendon just set up three ions on a date with seven compounds to see which compounds would work together strongly enough to extract into the coffee. DATA!Hendon displays his data in two ways - interaction lengths between ion and coffee compound and the relative binding energy between ion and coffee compound. Christine's Interpretation: You decide to chart your friends dates because it's getting complicated to keep all your info in multiple group texts. You notice that the shorter the date was, the better it went because they knew so quickly they liked each other they didn't even need to continue the first date (not real life, trying to make the metaphor work here people). So you chart the interaction length between friend and roommate. You also chart their "binding energy" with one another. . . I'll let you fill in the blanks on what that might mean. These results are shown below. (I took these data straight from Hendon et al., 2014 so mad credit and thanks). The simplest and most straightforward way to think about these data is that the shorter the interaction length, the stronger the pull. Similarly, the stronger the relative binding energy, the stronger the pull. When looking at table 1 and figure 3, the data are consistent. You can see that magnesium has both the shorter interaction length and the stronger relative binding energy across all coffee compounds. Magnesium has the best pull. This suggests that if we want to improve extraction then our water should have a higher proportion of magnesium. We've seen the data. We know that magnesium is going to improve our extraction Let's talk about how we can actually apply this data to making a better cup of coffee. Christine's interpretation: You realize your roommate Magnesium connected super well with all seven guy friends and now they all want to date her. SummaryThere's a few things we can take from Hendon et al. (2014), some of which couldn't be implied with the data shown in the paper. 1) Magnesium is favorable for extraction.
RESEARCH SHOWS MAGNESIUM IS GOOD FOR SPRO. Happy Brewing,
Jon Clutton Coffee Scientist/Barista
Comments
|
THE WILD WAY CONTRIBUTING WRITERS: |



 RSS Feed
RSS Feed